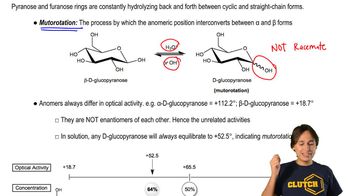
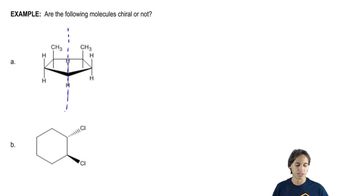
We predicted that the products would have a 1,2- or 1,4-relationship of the proper substituents. Draw the charge-separated resonance forms of the reactants to support these predictions.
(a) (b)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: