Multiple Choice
When monosaccharides form cyclic hemiacetals, which type of molecule is released during the linkage process?
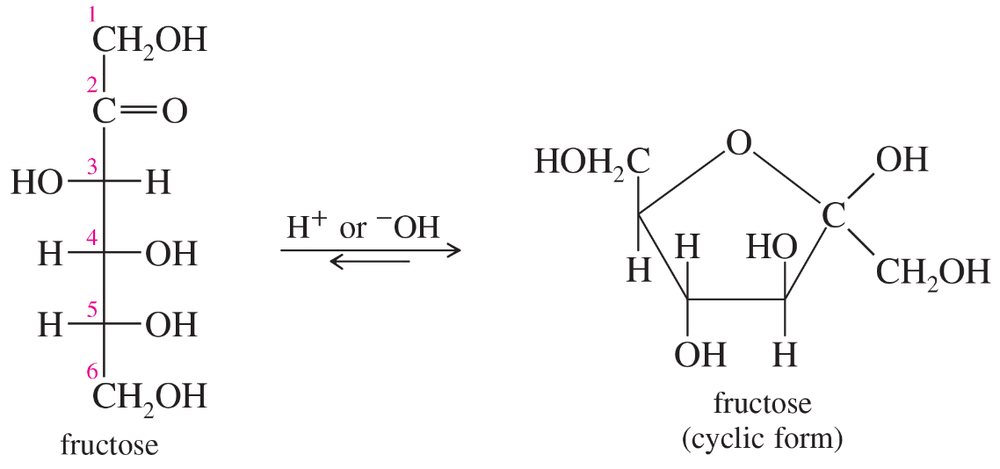
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 6:23m
6:23mMaster Monosaccharides - Forming Cyclic Hemiacetals with a bite sized video explanation from Johnny
Start learning