Predict the product of the following reactions. [Two of them are Williamson ether syntheses. Why isn't the other?].
(b)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:
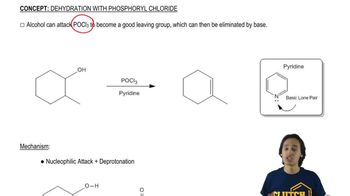

 3:50m
3:50mMaster The Mechanism of Williamson Ether Synthesis. with a bite sized video explanation from Johnny
Start learning