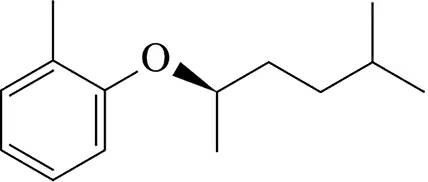
Two different Williamson ether syntheses can be used to make the compound in (a). Show them. The compound in (b), however, can only be made one way. Show it and explain why a second Williamson ether synthesis is not possible.
(a)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 3:50m
3:50mMaster The Mechanism of Williamson Ether Synthesis. with a bite sized video explanation from Johnny
Start learning