Without concerning yourself with the mechanism of the reaction, calculate the equilibrium constant for the following equilibrium processes. (Assume T = 298 K.)
(a)
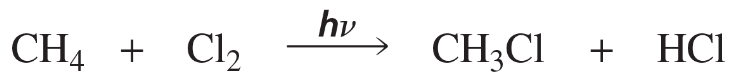
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: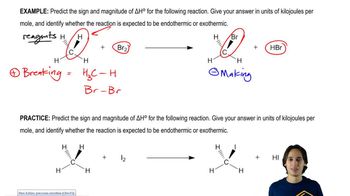
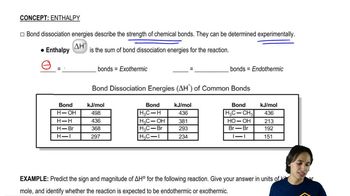

 4:09m
4:09mMaster How to calculate enthalpy using bond dissociation energies. with a bite sized video explanation from Johnny
Start learning