Textbook Question
Predict the product of the following sigmatropic rearrangements. Be sure to rationalize the stereochemical outcome with a chair-like transition state.
(d)
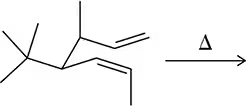
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: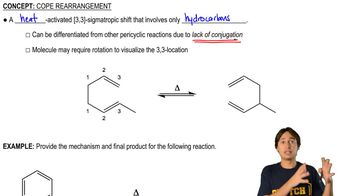
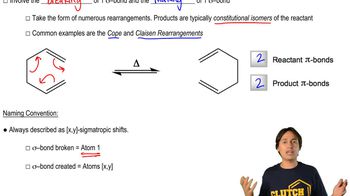
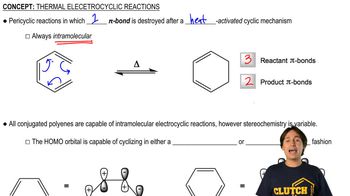

 6:28m
6:28mMaster Definition of Cope Rearrangement with a bite sized video explanation from Johnny
Start learning