For each pair, choose the nucleophile that would react most quickly in an SN2 reaction (assume H2O is the solvent).
(c)
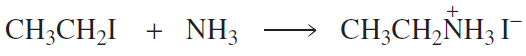
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: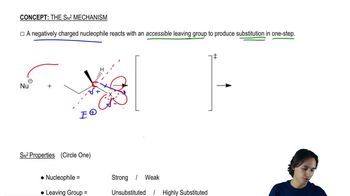
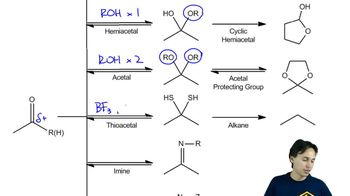

 8:33m
8:33mMaster Drawing the SN2 Mechanism with a bite sized video explanation from Johnny
Start learning