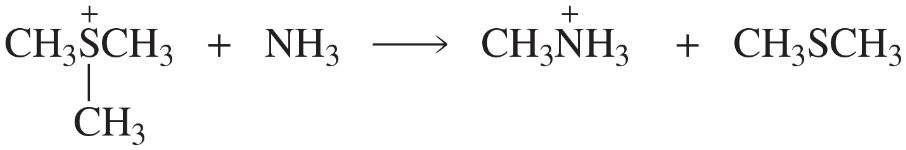
Practice your electron-pushing skills by drawing a mechanism for the following SN2 reactions.
(c)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 8:33m
8:33mMaster Drawing the SN2 Mechanism with a bite sized video explanation from Johnny
Start learning