Textbook Question
For each compound, predict the major product of free-radical bromination. Remember that bromination is highly selective, and only the most stable radical will be formed.
(f)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: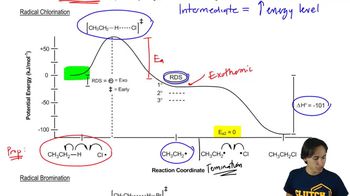
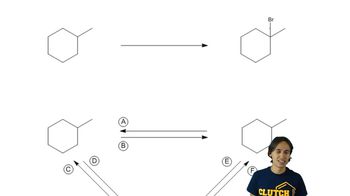

 1:08m
1:08mMaster Radical selectivity:Alcoholics Anonymous Version with a bite sized video explanation from Johnny
Start learning