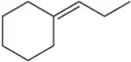
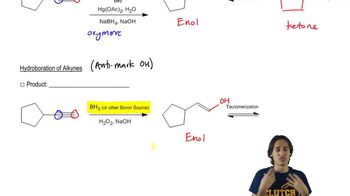
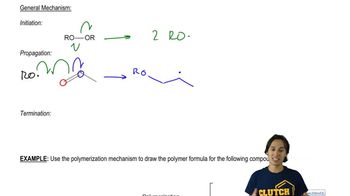
Predict the major products of the following reactions, and propose mechanisms to support your predictions.
(b)
HINT: Remember to write out complete structures, including all bonds and charges, when writing a mechanism or determining the course of a reaction.