Show how you would synthesize each compound using methylenecyclopentane as your starting material.
(b)
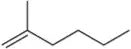
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:
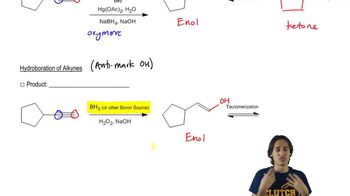

 1:18m
1:18mMaster Overview of Hydrohalogention. with a bite sized video explanation from Johnny
Start learning