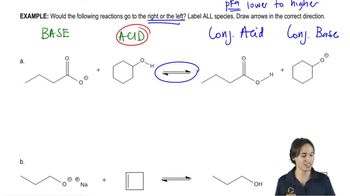
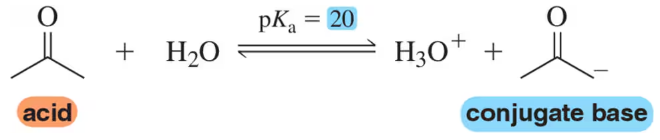Parts (a)–(d) of this assessment assist in the development of what will become a common theme in organic reactions and should be worked in order. [Think carefully about how each question relates to the others.]
(c) Without worrying about the mechanism of the reaction, estimate an equilibrium constant for the following carbonyl addition reaction based on the relative stability of the Lewis bases.