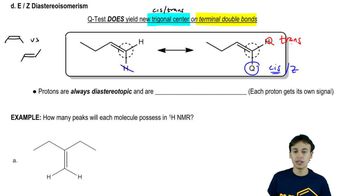
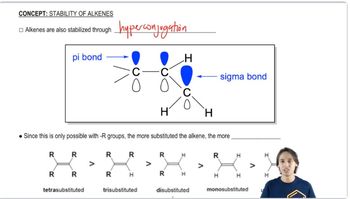
Which of the following cycloalkanes are capable of geometric (cis-trans) isomerism? Draw the cis and trans isomers.
b. 1-ethyl-3-methylcycloheptane
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: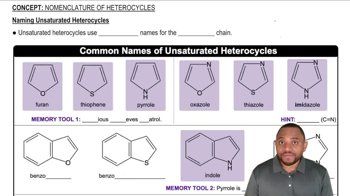

 4:28m
4:28mMaster How to name different types of double bonds or rings with a bite sized video explanation from Johnny
Start learning