Oxidative cleavages can help to determine the positions of the triple bonds in alkynes.
(a) An unknown alkyne undergoes oxidative cleavage to give adipic acid and two equivalents of acetic acid. Propose a structure for the alkyne.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: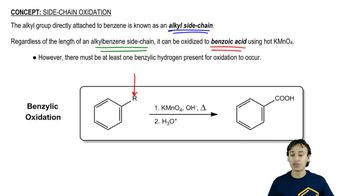


 2:13m
2:13mMaster General features of alkyne cleavage. with a bite sized video explanation from Johnny
Start learning