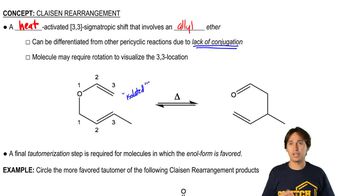
Determine whether each structure is likely to be colored or not. For those that you predict to be colored, indicate the extended conjugation by marking the series of continuous sp2 hybridized atoms.
(d)
(e)
(f)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: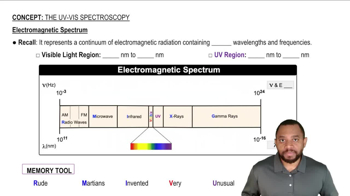


 5:29m
5:29mMaster Definition of Conjugation with a bite sized video explanation from Johnny
Start learning