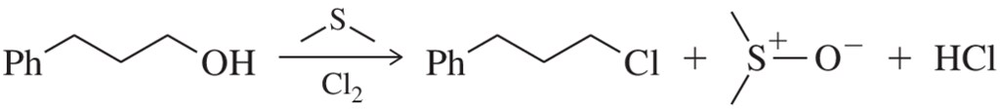
Two products are observed in the following reaction.
a. Suggest a mechanism to explain how these two products are formed.
b. Your mechanism for part (a) should be different from the usual mechanism of the reaction of SOCl2 with alcohols. Explain why the reaction follows a different mechanism in this case.