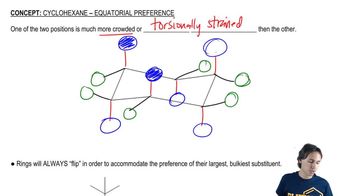
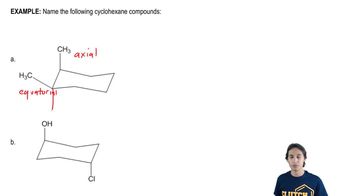
Draw 1,2,3,4,5,6-hexamethylcyclohexane with all the methyl groups
a. in axial positions.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

 1:05m
1:05mMaster What is a chair conformation? with a bite sized video explanation from Johnny
Start learning