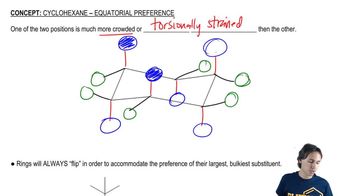
This is a Newman projection of a substituted cyclohexane.
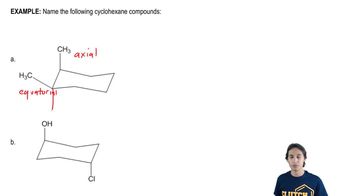
b. Draw the equivalent structure using wedge and dash notation on a cyclohexane hexagon.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

 1:05m
1:05mMaster What is a chair conformation? with a bite sized video explanation from Johnny
Start learning