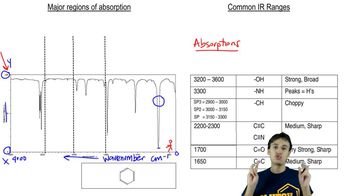
Rank the following compounds from highest wavenumber to lowest wavenumber for their C=O absorption bands:
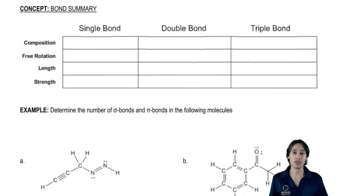
b.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: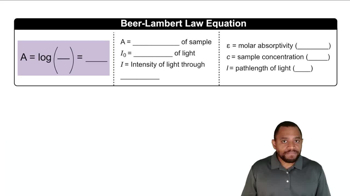

 16:4m
16:4mMaster General Features of IR Spect with a bite sized video explanation from Johnny
Start learning