The following reaction takes place several times faster than the reaction of 2-chlorobutane with HO-:
b. Explain why the OH group in the product is not bonded to the carbon that was bonded to the Cl group in the reactant.
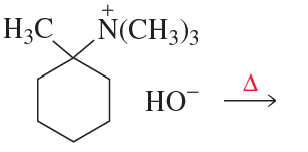 .
. Verified step by step guidance
Verified step by step guidance

