Textbook Question
A radical reaction, as it progressed through its propagation steps, involved the following radical species. Suggest which products might form in all possible termination steps (six products are possible).
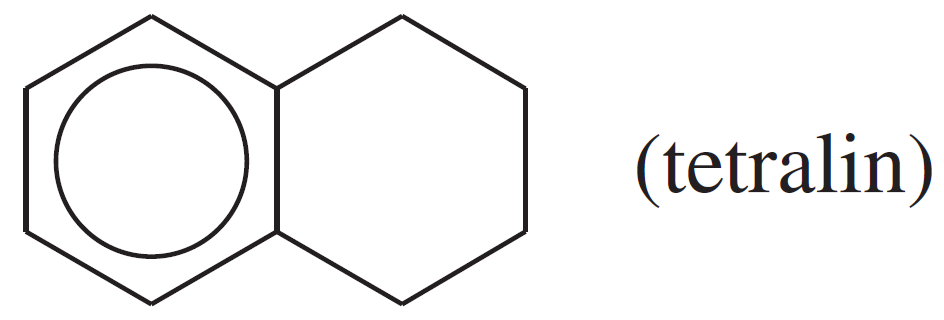
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: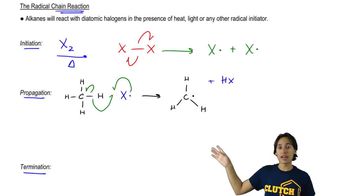

 6:14m
6:14mMaster Side-Chain Halogenations with a bite sized video explanation from Johnny
Start learning