Textbook Question
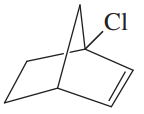
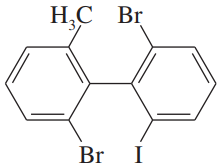
Draw three-dimensional representations of the following compounds. Which have asymmetric carbon atoms? Which have no asymmetric carbons but are chiral anyway? Use your models for parts (a) through (d) and any others that seem unclear.c. ClHC═C═C(CH3)21-chloro-3-methylbuta-1,2-diened. ClHC═CH―CH═CH21-chlorobuta-1,3-diene