Propose mechanisms consistent with the following reactions.
(d)
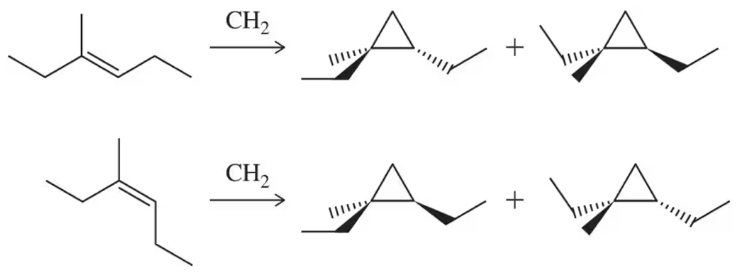
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: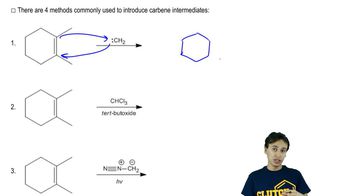


 1:49m
1:49mMaster General properties of cyclopropanation. with a bite sized video explanation from Johnny
Start learning