Answer the following questions for each compound:
a. How many signals are in its 13C NMR spectrum?
b. Which signal is at the lowest frequency?
9. CH2=CHBr
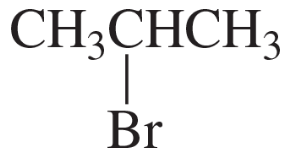
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: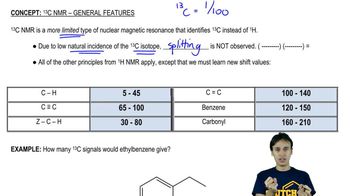

 4:m
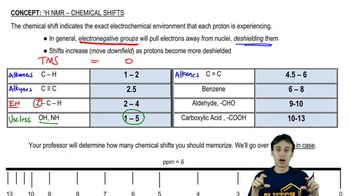
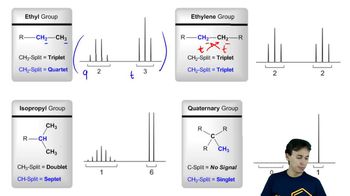
4:mMaster 13C NMR General Features with a bite sized video explanation from Johnny
Start learning