a. When a carboxylic acid is dissolved in isotopically labeled water (H2O18) and an acid catalyst is added, the label is incorporated into both oxygens of the acid. Propose a mechanism to account for this.
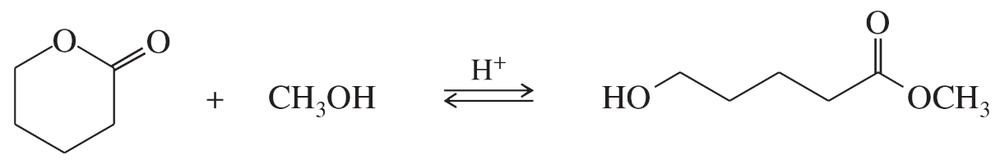
c. If an ester is dissolved in isotopically labeled water (H2O18) and an acid catalyst is added, where will the label reside in the product?