Draw the resonance contributors of the cyclooctatrienyl dianion.
b. Which of the resonance contributors makes the smallest contribution to the hybrid?

 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:
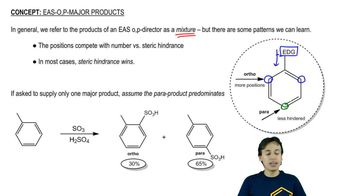
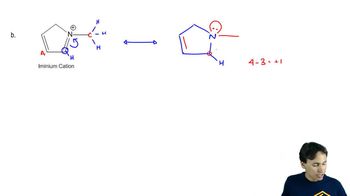

 3:34m
3:34mMaster The rules you need for resonance: with a bite sized video explanation from Johnny
Start learning