For each of the following substituents, indicate whether it withdraws electrons inductively, donates electrons by hyperconjugation, withdraws electrons by resonance, or donates electrons by resonance.
a. Br
b. CH2CH3
c.
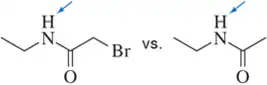
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 3:15m
3:15mMaster Why we need factors affecting acidity and when to use them. with a bite sized video explanation from Johnny
Start learning