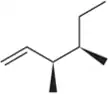
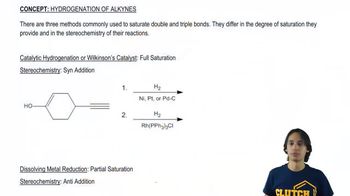
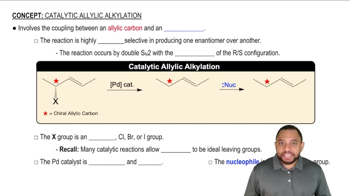
What product results when the following molecules are treated with H₂ Pd/C? Be sure to indicate the relative stereochemical outcome. Draw both enantiomers of any racemic mixtures. [It is difficult to control the stoichiometry of gases so there is enough H₂ to reduce all alkenes.]
(b)