Show how you would convert propan-1-ol to the following compounds using tosylate intermediates. You may use whatever additional reagents are needed.
c. CH3CH2CH2OCH2CH3, ethyl proyl ether
d. CH3CH2CH2CN, butyronitrile
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: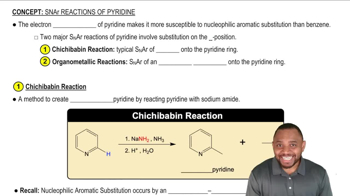

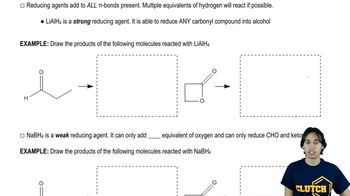

 7:53m
7:53mMaster Learning the mechanism of Sulfonyl Chlorides. with a bite sized video explanation from Johnny
Start learning