Given the atoms involved and the number of valence electrons remaining, complete the Lewis structure by placing bonds between atoms such that each has a full octet.
(a)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: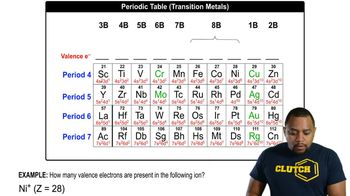
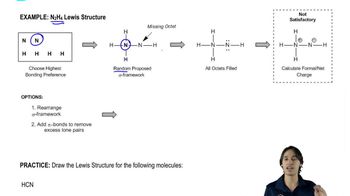


 3:49m
3:49mMaster How to use Organic Chemistry to make Lewis Structures easier. with a bite sized video explanation from Johnny
Start learning