Complete the following multistep syntheses using tosylate formation as one of the steps. The optimum number of steps for each synthesis is shown.
(a)
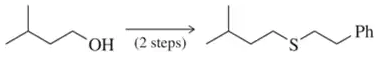
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 7:53m
7:53mMaster Learning the mechanism of Sulfonyl Chlorides. with a bite sized video explanation from Johnny
Start learning