Textbook Question
Using an appropriate tosylate intermediate, synthesize the following molecules starting from the appropriate alcohol.
(b)
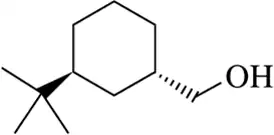
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:



 7:53m
7:53mMaster Learning the mechanism of Sulfonyl Chlorides. with a bite sized video explanation from Johnny
Start learning