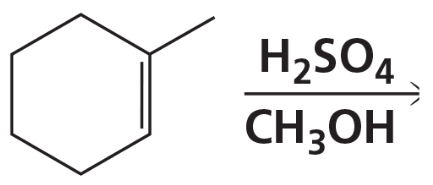
a. Draw the product or products that will be obtained from the reaction of cis-2-butene and trans-2-butene with each of the following reagents. If a product can exist as stereoisomers, show which stereoisomers are formed.
8. CH3OH + H2SO4
b. With which reagents do the two alkenes react to form different products?