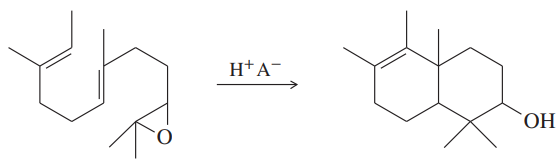a. Propose a mechanism for the conversion of cis-hex-3-ene to the epoxide (3,4-epoxyhexane) and the ring-opening reaction to give the glycol, hexane-3,4-diol. In your mechanism, pay particular attention to the stereochemistry of the intermediates and products.
b. Repeat part (a) for trans-hex-3-ene. Compare the products obtained from cis- and trans-hex-3-ene. Is this reaction sequence stereospecific?