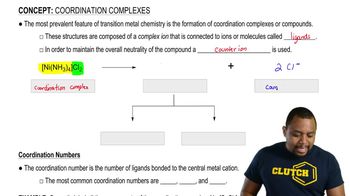
Treatment of tert-butyl alcohol with concentrated HCl gives tert-butyl chloride.
When the concentration of H+ is doubled, the reaction rate doubles. When the concentration of tert-butyl alcohol is tripled, the reaction rate triples. When the chloride ion concentration is quadrupled, however, the reaction rate is unchanged. Write the rate equation for this reaction.