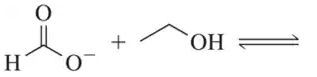
(a) On a reaction coordinate diagram, show why a general, one-step reaction is faster when a catalyst is employed.
(b) Why does a catalyst often allow a reaction to proceed at lower temperatures?
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 6:07m
6:07mMaster Introduction to free energy diagrams. with a bite sized video explanation from Johnny
Start learning