Show how the following amino acids might be formed in the laboratory by reductive amination of the appropriate α-ketoacid.
(a) alanine
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: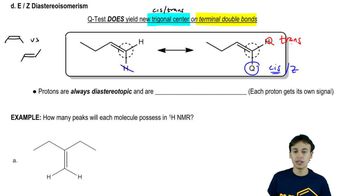
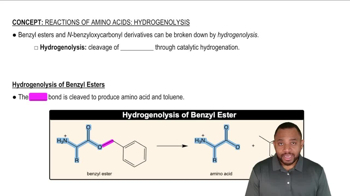
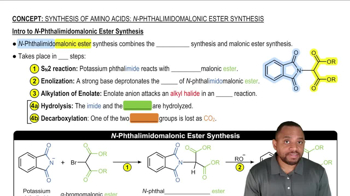

 9:34m
9:34mMaster Peptides and Polypeptides with a bite sized video explanation from Johnny
Start learning