Which diene and which dienophile could be used to prepare each of the following?
e.
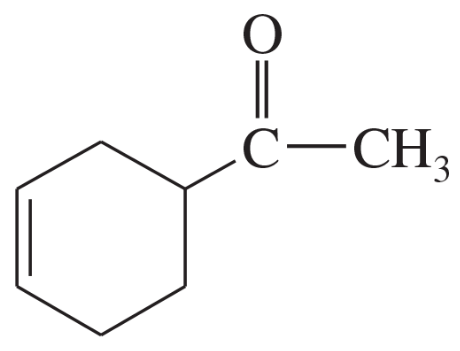
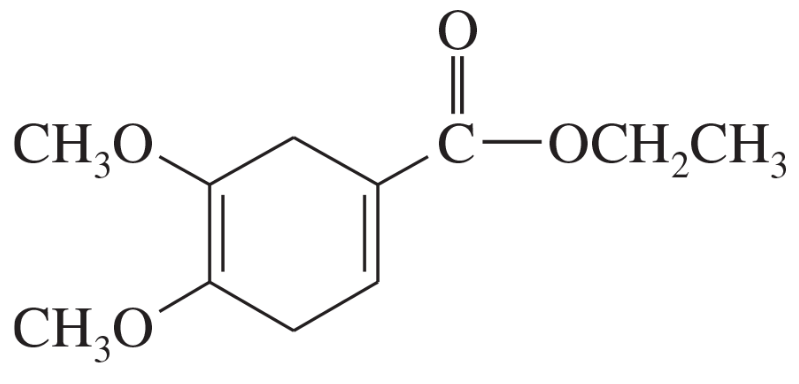
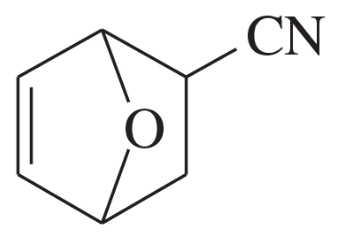
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:
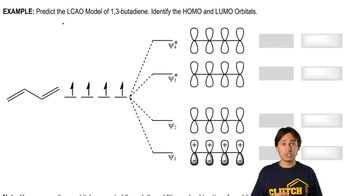
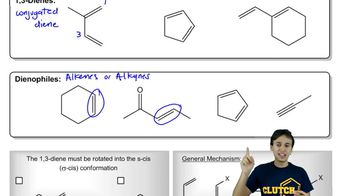

 4:02m
4:02mMaster Diels-Alder Retrosynthesis with a bite sized video explanation from Johnny
Start learning