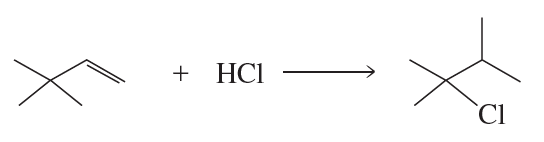
A wayward chemist proposed the following mechanism for the addition of HBr to an alkene.
(a) Why is this mechanism unlikely?
(b) Compare the reaction coordinate diagrams for the actual mechanism studied in Section 8.3.1 and this alternate mechanism on the same graph.