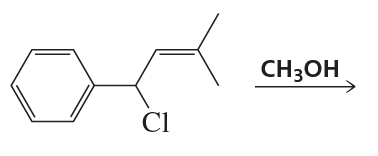
a. Draw the structures of the products obtained from the reaction of each enantiomer of cis-1-chloro-2-isopropylcyclopentane with sodium methoxide in methanol.
b. Are all the products optically active?
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 2:27m
2:27mMaster Overview of the flowchart. with a bite sized video explanation from Johnny
Start learning