Which is a stronger acid?
c. CH3OCH2CH2CH2OHorCH3CH2OCH2CH2OH
d.
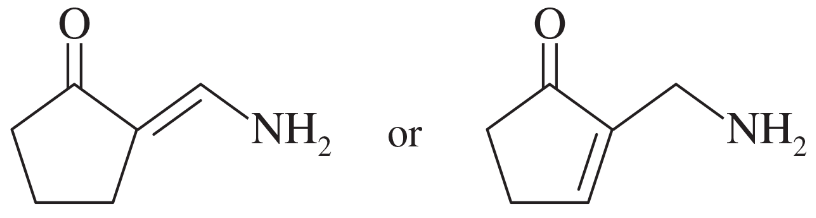
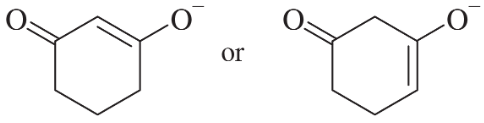
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 3:15m
3:15mMaster Why we need factors affecting acidity and when to use them. with a bite sized video explanation from Johnny
Start learning