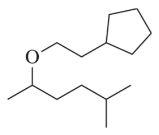
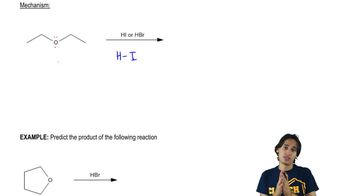
A good Williamson synthesis of ethyl methyl ether would be
What is wrong with the following proposed synthesis of ethyl methyl ether? First, ethanol is treated with acid to protonate the hydroxy group (making it a good leaving group), and then sodium methoxide is added to displace water.