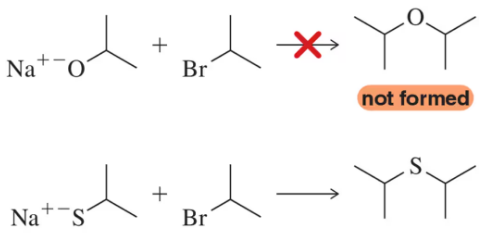
(a) Show how ethanol and cyclohexanol may be used to synthesize cyclohexyl ethyl ether (tosylation followed by the Williamson ether synthesis).
(b) Why can't we synthesize this product simply by mixing the two alcohols, adding some sulfuric acid, and heating?