Each of the following reactions has two nucleophiles that could add to the intermediate formed by the reaction of the alkene with an electrophile. What is the major product of each reaction?
c.
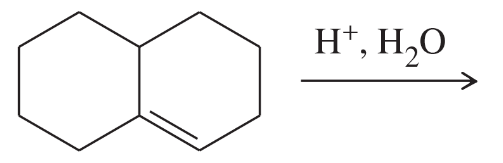
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:



 6:32m
6:32mMaster General properties of acid-catalyzed hydration. with a bite sized video explanation from Johnny
Start learning