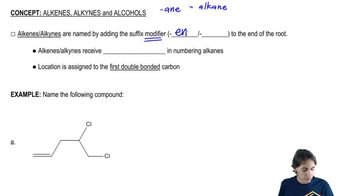
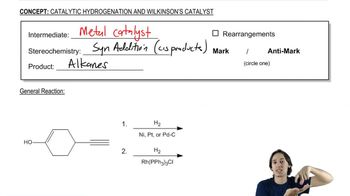
We have studied a number of pericyclic reactions previously. Draw the mechanism of the steps shown. The section number where this material was first studied is given for your review.
(a)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: