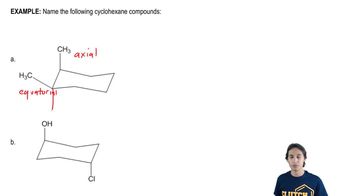
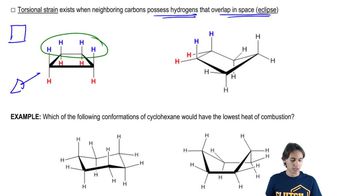
For each structure shown, draw the two chair conformations and choose which is most stable. Be sure that your second chair is the flipped version of the first. [Make sure that wedged substituents are up in the chair, regardless of whether up is equatorial or axial.]
(g)