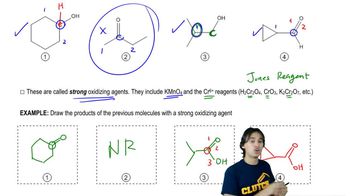
There are two mechanisms by which each of the two enantiomers can form in the reaction shown in Figure 9.37. Show them.
<IMAGE>
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 4:34m
4:34mMaster Acid-Catalyzed Epoxide Ring-Opening with a bite sized video explanation from Johnny
Start learning