Multiple Choice
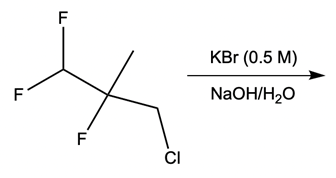
Which of the following compounds would you expect to have the greatest relative rate of reaction?
 Verified step by step guidance
Verified step by step guidance
 2:31m
2:31mMaster Nucleophilic Catalysis Concept 1 with a bite sized video explanation from Johnny
Start learning
