Make models of the following compounds, and predict the products formed when they react with the strong bases shown.
(c) (d,l)-1,2-dibromo-1,2-diphenylethane + (CH3CH2)3N:
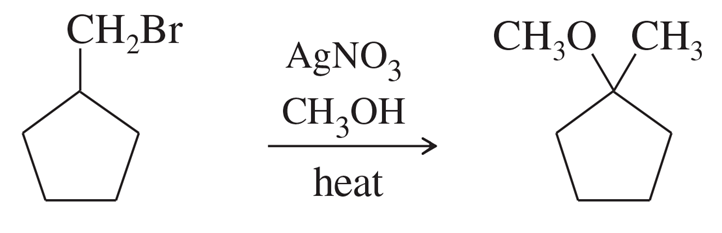
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 2:27m
2:27mMaster Overview of the flowchart. with a bite sized video explanation from Johnny
Start learning