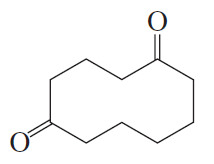
In contact with a platinum catalyst, an unknown alkene reacts with three equivalents of hydrogen gas to give 1-isopropyl-4-methylcyclohexane. When the unknown alkene is ozonized and reduced, the products are the following:
Deduce the structure of the unknown alkene.